Researchers at UofL have discovered a way to revitalize cone receptors that have deteriorated as a result of retinitis pigmentosa (RP). Working with animal models, Henry J. Kaplan, MD, and a group of researchers in the Department of Ophthalmology and Visual Sciences discovered that replenishing glucose under the retina and transplanting healthy rod stem cells into the retina restore function of the cones.
The research, conducted by Kaplan, chair of the Department of Ophthalmology and Visual Sciences, Douglas Dean, PhD, and Wei Wang, PhD, and published in December in Cell Reports, could lead to therapies for preserving or recovering central vision in patients with RP. Kaplan will present the research findings at five conferences in the United States and abroad beginning this month.
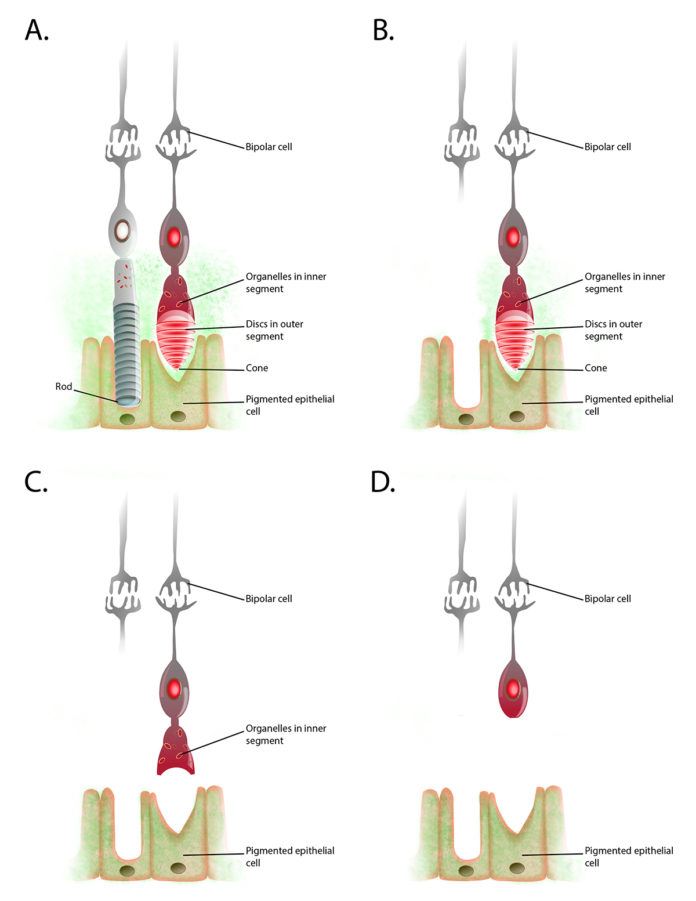
Retinitis Pigmentosa is an inherited disease in which the photoreceptor cells in the retina – rods and cones – deteriorate over time. Photoreceptors absorb and convert light into electrical signals, which are sent through the optic nerve to the brain. Rods, located in the outer regions of the retina, allow peripheral and low-light vision. Cones, located mostly in the central part of the retina, allow perception of color and visual detail.
In RP, rods deteriorate first, causing the peripheral and low light vision loss typically associated with the disease. In later stages, the cones also deteriorate. Without cone function, RP patients lose the high-resolution daylight vision necessary for reading, facial recognition and driving. As a result, this stage of RP vision loss is more debilitating than the loss of nighttime or peripheral vision. RP affects 1 in 4,000 people globally.
Recent research has shown that as the rods deteriorate, the cones are no longer able to access glucose, which becomes trapped in the retinal pigment epithelium (RPE). As a result of glucose starvation, the cones go dormant and eventually die.
The UofL researchers found that the cones remain dormant for a period of time before they are completely lost, and if the glucose supply can be replenished during dormancy, the cones can be regenerated. The researchers were able to successfully restore cone access to glucose in either of two procedures. First, by transplanting rod-specific induced pluripotent stem cells beneath the retina, and second by injecting glucose directly into the subretinal space.
“Following rod stem cell transplant, we observed reassembly of the cone inner segments, regeneration of cone outer segments and increased electrophysiologic function within 1,000 microns from the transplant margin for at least three months after the transplantation in all directions,” Kaplan said. “However, the recognition that glucose starvation of cones occurred because of the trapping of glucose in the RPE provides multiple new possible treatments to restore lost central vision including drug therapy, gene editing and regenerative medicine.”

Kaplan will present these findings at the 6th China Ocular Microcirculation Society Annual Meeting – International Ophthalmology Conference, Beijing, China, and the American Society of Retina Specialists, Boston, this month, at the Indiana Academy of Ophthalmology, Carmel, in September, the Retina Society, Boston, in October, and the 5th World Integrative Medicine Congress, Guangzhou, China in December.
This research has the potential to lead to therapies that preserve or restore central vision for individuals with RP.
“If therapy can prevent or reverse the onset of cone degeneration within the macula, most patients would be immeasurably helped and able to live a normal life despite the loss of peripheral vision and decreased dark adaptation,” Kaplan said.





























